Abstract
INTRODUCTION: The chronic MPN, essential thrombocytosis (ET), polycythemia vera (PV) and primary myelofibrosis (PMF), share mutations of JAK2, MPL and CALR, but vary widely with regard to sex distribution, age at diagnosis, disease evolution and outcomes. Given that age at diagnosis influences presenting phenotype, disease progression and clinical outcome, the impact of sex remains undefined. The aim of our study was to evaluate the impact of sex in presenting phenotype, disease progression, complications and mortality in a well-defined, prospective observational cohort.
PATIENTS AND METHODS: 630 individuals with ET, PV or PMF were enrolled in our observational cohort between 2005-2015. Median disease duration at the time of evaluation was 7.23 years, whereas 152 were enrolled within one year of diagnosis. All individuals were genotyped for neutrophil JAK V617F variant allele frequency (VAF), and JAK2 V617F-negative individuals were studied for other JAK2 mutations, CALR or MPL mutations. Multivariable logistic regression was used to evaluate associations of sex, age and molecular characteristics with presenting phenotype; multivariable cox regression was used to evaluate associations of sex, age, presenting phenotype, and molecular characteristics with mortality, and survival curves were generated using the Kaplan Meier method.
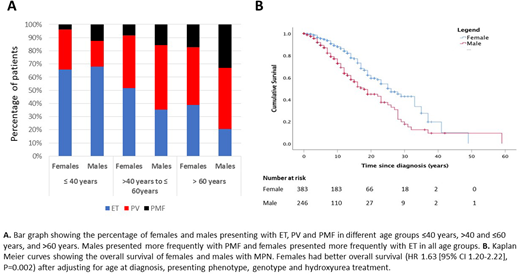
RESULTS: The cohort comprised 630 individuals (246 males and 384 females) with 6819 patient-years of follow up. Over a median follow up of 9 (Q25-75% 6, 15) years, 185 individuals died. Males had a higher mean age of presentation (52.9 versus 48.8 years, P=0.002). Compared with females, males were more likely to present with PMF than PV or ET (P <0.001), and this sex difference remained statistically significant across JAK2 V617F positive and negative groups, and across age groups (≤40 years, >40 and ≤60 years, and >60 years) (Figure 1A). In a multivariable logistic regression model, male sex was significantly associated with PMF as presenting phenotype (P<0.001) after adjusting for age at diagnosis (P=0.4), neutrophil and peripheral blood CD34+ JAK2 V617F VAF (P=0.729 and P=0.064 respectively). Females had a higher rate of venous thromboembolism (15.7% vs. 6.9%, P<0.001) while males had higher rates of progression to acute myeloid leukemia (6.5% vs. 2.9%, P=0.028) and overall mortality (38.2% vs. 23.7% P<0.001). In a multivariable cox regression model, male sex was significantly associated with increased mortality (HR 1.63 [95% CI 1.20-2.22], P=0.002) after adjusting for age at diagnosis (HR 1.10 [95% CI 1.08-1.12), P<0.001), presenting phenotype (PV, HR 1.66 [95% CI 1.13-2.45], P=0.010; PMF 3.70 [95% CI 2.46-5.57), P <0.001), genotype (JAK2V617F+ reference category, CALR HR 0.83 [955 CI 0.49-1.40], P=0.064; MPL HR 0.74 [95% CI 0.23-2.35], P =0.493; triple negative HR 2.72 [95% CI 1.30-5.69], P=0.008), and hydroxyurea therapy (HR 1.00 [95% CI 0.73-1.36], P=0.992). The association of male sex with higher mortality was also seen in subgroup analyses of the JAK2 V617F-positive and JAK2 V617F-negative groups separately (Figure 1B).
CONCLUSIONS: Males with MPN have inferior survival compared to females with MPN, even when adjusted for age at presentation, presenting phenotype, and molecular characteristics. Further, males present more frequently with PMF while females present more frequently with ET. These striking sex-based differences in MPN presentation and outcomes demonstrate that male sex is associated with more aggressive disease biology independent of other factors, and further study to dissect the molecular mechanisms are warranted. Moreover, sex stratification should be considered in MPN prognostic risk algorithms and when designing and interpreting results from clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal